Answers
Answer:
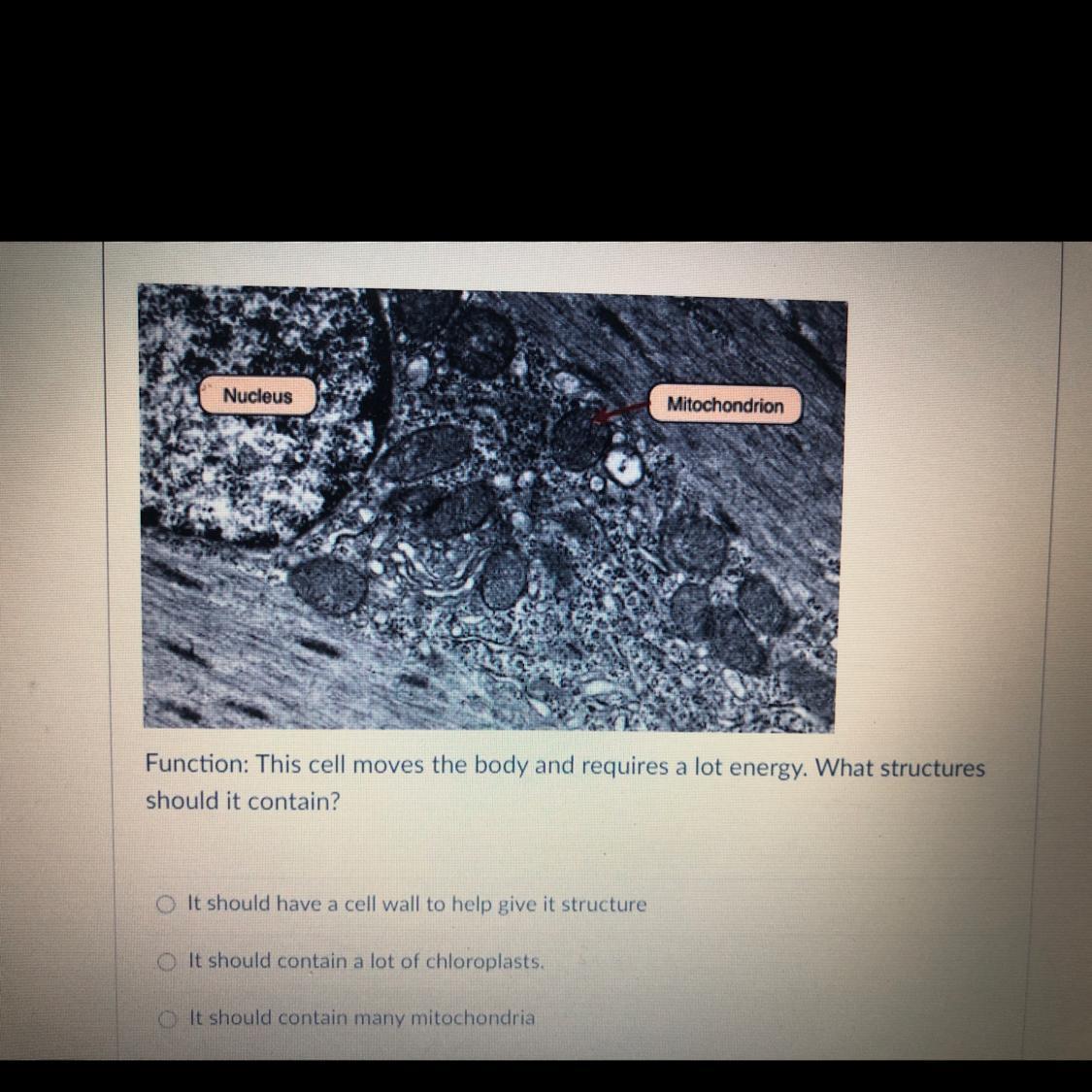
A. it should have a cell wall to give it structure
Related Questions
Using Reaction A, how many grams of CO2 can be created from 5.67 moles of water?
127.6 g CO2
199.6 g CO2
81.65 g CO2
311.85 g CO2
Answers
Answer:
81.65 g CO2
Explanation:
The primary forces of attraction between water molecules in H2O(l) are
1.
ionic bonds
2.
hydrogen bonds
3.
molecule-ion attractions
4.
van der Waals forces
Submit Answer
Answers
Answer:
2. Hydrogen Bonds
Explanation:
Since water is a polar covalent molecule, there is a slight negative and positive end. Due to this, the oxygen end of one water molecule gravitates towards the hydrogen molecules of another water molecule. This accounts for a bunch of weird properties of water, like why ice floats. It's also what makes water the "universal solvent," and gives all life on earth the ability to even exist.
The primary forces of attraction between water molecules in H₂O (I) are hydrogen bonds.
What kind of chemical bonding is present in water molecule?Hydrogen bonding is present in water molecule due to which it exhibits an excellent property of adhesion to itself and to other substances.The hydrogen bonding is a result of electrostatic forces of attraction which are generated by the difference in charge between slightly positive hydrogen ions and slightly negative other ions.
In case of water,hydrogen bonds are formed between neighboring hydrogen and oxygen atoms of the nearby water molecules.The attraction between water molecules itself results in a formation of a bond called as a hydrogen bond.
It is a type of covalent bond which is formed between hydrogen and oxygen atoms as one oxygen atom shares its two electrons with two hydrogen atoms .The positive charge of one hydrogen atom associates with negative charge of oxygen atom.These are weak interactions which are formed between a hydrogen atom each with a partial positive charge and an oxygen atom which is more electronegative than hydrogen.
To learn more about bonding in water click here:
https://brainly.com/question/5302822
#SPJ2
How does carbonic acid work to maintain blood pH? (Select all that apply.) Check All That Apply When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood. When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood. When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood. When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood. Carbonic acid is added to the blood until the pH reaches 7.4. Carbonic acid is added to the blood until the pH reaches 7.4. Carbonic acid always lowers the blood pH to 7.4. Carbonic acid always lowers the blood pH to 7.4. Carbonic acid can raise or lower the pH of blood. Carbonic acid can raise or lower the pH of blood.
Answers
Answer:
When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood.
When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood.
Carbonic acid can raise or lower the pH of blood.
Explanation:
A buffer is a solution that resists changes to its pH when small quantities of acids or bases are added to it. The human blood serves as a buffer as it contains a buffer of carbonic acid (H2CO3) and bicarbonate anion (HCO3-) which serves to maintain blood pH between 7.35 and 7.45. Other buffering systems in blood exist such as the Hydrogen ion and oxygen gas which affects oxygen binding to haemoglobin, however the carbonic-acid-bicarbonate buffer is the most important buffer for maintaining acid-base balance in the blood.
A buffer solution is made up of an acid and its conjugate base or a base and its conjugate acid. For carbonic acid-bicarbonate buffer, carbonic acid serves as the acid while bicarbonate serves as the base. When a little quantity of a base as hydroxide ions is added to a buffer, the acid reacts with it and remove it from the solution. On the other hand, when a little quantity of an acid as hydrogen ions are added to a buffer, the conjugate base reacts with it and remove it from the solution, thus keeping the pH of the solution fairly constant.
In the carbonic acid-bicarbonate buffer:
When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood.
When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood.
Thus, carbonic acid can raise or lower the pH of blood.
Carbonic acid work to maintain blood pH as follows:
When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood.When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood.Carbonic acid can raise or lower the pH of blood.WHAT IS BUFFER SOLUTION:A buffer is a solution that resists changes to its pH when small quantities of acids or bases are added to it. A buffer is made up of an acid and its conjugate base or a base and its conjugate acid. Carbonic acid is an example of buffer that contains an acid with it's conjugate base.This means that, carbonic acid works to maintain blood pH as follows:
When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood.When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood.Carbonic acid can raise or lower the pH of blood.Learn more about buffers at: https://brainly.com/question/24188850
How many watts do I consume if I eat a 600 Calorie sandwich in 600 seconds
Answers
Answer:
1 watt is conduned by you ok
Base your answer on the equation and diagram below represent an electrochemical cell at 298 K and 1 atmosphere.
When the switch is closed, electrons flow from
A) Ag+(aq) to Mg2+(aq)
B) Mg(s) to Ag(s)
C) Ag(s) to Mg(s)
D) Mg2+(aq) to Ag+(aq)
Answers
When the switch is closed, electrons flow from the solid magnesium electrode, Mg(s) to solid silver electrode, Ag(s).
Electronegativity of metals
Electronegativity of metals refers to the ability of the atoms of metallic elements to attract electrons from the other metallic elements.
Electronegativity increases down the activity series.
Silver (Ag) will have more tendency to attract electron more than magnesium (Mg).
Thus, when the switch is closed, electrons flow from the solid magnesium electrode, Mg(s) to solid silver electrode, Ag(s).
Learn more about electronegativity here: https://brainly.com/question/24977425
#SPJ2
Can someone plss help me answer those 4 questions by tonight.Thank you !
Answers
Answer:
no problem hey I will help you
what will the sun become when it dies
Answers
Answer:
It will become a red giant
Explanation:
When the sun runs out of its hydrogen fuel and the hydrogen atoms are combined together to make helium atoms, it expands into a red giant, which is made up of helium atoms and gases.
A student is asked to determine the identity of an unknown metal. The student decides to use a calorimetry experiment to find the specific heat. The student will use this as a means of identifying the metal Which of the following measurements cannot made using standard laboratory equipment?
A mass of water
B temperature of metal
с heat lost by metal
D mass of metal
Answers
Answer:
Temperture of the medal
Explanation:
Bc ik
Can you tell me any chemical reaction that occur due to kinetic energy
Answers
Answer:
The molecules in gasoline (octane, the chemical formula shown) contain chemical energy. This energy is transformed into kinetic energy that allows a car to race on a racetrack.
A solution of dispersant is made by taking 15.0 mL of a 50.0 mg/mL solution of Randyne and mixing it with 50.0 mL of water. Calculate the final concentration of the Randyne in this solution, in units of grams per milliliter.
Answers
Answer:
The final concentration of the Randyne in grams per milliliter = 0.011 g/mL
Explanation:
As we know
C1V1 = C2V2
C1 and C2 = concentration of solution 1 and 2 respectively
V1 and V2 = Volume of solution 1 and 2 respectively
Substituting the given values, we get -
[tex]50 * 15 = X * (15+50)\\X = 11.54[/tex] mg/mL
The final concentration of the Randyne in grams per milliliter = 0.011 g/mL
The final concentration of the Randyne in this solution is 0.01 g /mL.
How to calculate dilutions?It is very important to know the dilution methods in a chemistry lab. The dilution from the stock solution can be prepared by using the formula,
[tex]C_1V_1 = C_2V_2[/tex]
Where,
[tex]C_1[/tex]- concentration of the stock solution
[tex]V_1[/tex] - the volume of the stock solution
[tex]C_2[/tex] - concentration of the diluted solution
[tex]V_2[/tex] - the volume of diluted solution
Put the values in the formula,
[tex]50 \times 15 = C_2 \times 75 \\\\C_2 = \dfrac {750}{75 }\\\\C_2 = 10{\rm \ mg/mL \ \ \ or} \\\\ C_2 = 0.01 \rm \ g/mL[/tex]
Therefore, the final concentration of the Randyne in this solution is 0.01 g /mL.
Learn more about dilution methods:
https://brainly.com/question/25307719
A catalyst is:
a chemical found in leaves
a chemical which promotes a chemical reaction
a chemical which reacts with sunlight
a cell with chlorophyll
Answers
Answer:
a chemical which promotes a chemical reaction
and sppeds up that reaction.
What is science..
No Spam
Answers
Answer: science is a systematic enterprise that builds and organizes knowledge in the form of testable explanations and predictions about the universe
Explanation:
Answer:
Science is a very Idiot subject and doing dimag ka dahi xD
What is a cell? ♀️
Answers
Answer:
Cell is defined as the smallest unit or basic unit of life.
the smallest part of a living thing that can carry out the activities needed for life. Basically the unit of all forms of life.
Explanation:
hope this helps in anyway :)
A solution with a pH of 5.30 has a H+ concentration of
Answers
Answer:
5.01 x 10^-6 M
Explanation:
PH= -log [H+]
[H+] = 10^-PH
Help plz:)))I’ll mark u Brainliest
Answers
Answer:
2.475 mol of O2 formed.
Explanation:
Given 1.65 moles of KClO3 as the target amount in the reactant, used the coefficient of the balanced chemical reaction involved to determine the number of moles of O2 molecules formed.
x mole of O2 = 1.65 mol KClO3 x [(3 mol O2)/ (2 mol KClO3)] = 2.475 mol of O2
x mole of O2 formed = 2.475 mol of O2
Which of the following compounds would have the lowest
solubility?
ОНczА
O Nm3R
O Bv3(AX)2
O Hn(EX2)2
O MRD2
Answers
You studied the body of someone who died in their sleep and found that your assistant accidentally left the body facedown while you were away. Knowing that the person, in life, slept face-up, you can see that the blood is pooled at their backside. What has your assistant accidentally revealed about the victim?
A. The victim probably died only a few hours before the assistant’s accident
B. The victim was probably poisoned to death
C. The victim died at least 8 hours before the body was flipped
D. The victim was probably moved after they had died by someone else—or they didn’t die in their sleep!
Answers
Answer:
D
Explanation:
TRUE or FALSE: We have 2 copies of each gene because we have 2 parents.
Answers
How does artificial selection change a population over time?
Answers
You can use quillbot.com to reword it
please help ME WITH CHEM I DONT HAVE ENOUGH TIME! calculate the number of hydrogen molecules that it would have contained at stp if it had a volume of 200,00m cubed.
Answers
We know that, at STP, one mole of a gas occupies 22.4 L of volume
we'll use the same principle to solve this problem
Converting given volume to Liters:
We know that 1 m³ = 1000 L
So, 20000 m³ = (20000)*(1000) L = 2 * 10⁷ L
Converting Liters to moles:
As mentioned above, at STP, one mole occupies 22.4 Liters
Number of moles in 2 * 10⁷ L = 2 * 10⁷ / 22.4
Number of moles = 8.9 * 10⁵ moles
Converting moles to number of particles:
We know that 1 mole contains 6.022 * 10²³ molecules
So, 8.9 * 10⁵ moles contain [(8.9 * 10⁵) * (6.022 * 10²³)] molecules
Number of molecules = 53.6 * 10²⁸ molecules
In proper scientific notation:
Number of molecules = 5.36 * 10²⁹ molecules
Carbon-14 has a half-life of 5730 years. How much of a 144 g sample of carbon-14 will remain after 100,000 years?
Answers
Answer:
0.001 g
Explanation:
From the question given above, the following data were obtained:
Half-life (t½) = 5730 years
Original amount (N₀) = 144 g
Time (t) = 100,000 years
Amount remaining (N) =?
Next, we shall determine the number of half-lives that has elapsed. This can be obtained as follow:
Half-life (t½) = 5730 years
Time (t) = 100,000 years
Number of half-lives (n) =?
n = t / t½
n = 100,000 / 5730
n ≈ 17
Finally, we shall determine the amount remaining. This can be obtained as follow:
Original amount (N₀) = 144 g
Number of half-lives (n) = 17
Amount remaining (N) =?
N = 1/2ⁿ × N₀
N = 1/2¹⁷ × 144
N = 1/131072 × 144
N = 0.000007 × 144
N ≈ 0.001 g
Thus, the amount remaining after 100000 years is 0.001 g
In the coal-gasification process, carbon monoxide is converted to carbon dioxide via the following reaction: CO (g) H2O (g) CO2 (g) H2 (g) In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00-L reaction vessel. At equilibrium, there were 0.19 mol of CO remaining. Keq at the temperature of the experiment is __________. A) 5.47 B) 1.0 C) 1.78 D) 0.75 E) 0.56
Answers
Answer: [tex]K_{eq}[/tex] at the temperature of the experiment is 0.56.
Explanation:
Moles of [tex]CO[/tex] = 0.35 mole
Moles of [tex]H_2O[/tex] = 0.40 mole
Volume of solution = 1.00 L
Initial concentration of [tex]CO[/tex] = [tex]\frac{0.35mol}{1.00L}=0.35M[/tex]
Initial concentration of [tex]H_2O[/tex] = [tex]\frac{0.40mol}{1.00L}=0.40M[/tex]
Equilibrium concentration of [tex]CO[/tex] = [tex]\frac{0.19mol}{1.00L}=0.19M[/tex]
The given balanced equilibrium reaction is,
[tex]CO(g)+H_2O(g)\rightleftharpoons CO_2(g)+H_2(g)[/tex]
Initial conc. 0.35 M 0.40 M 0 M 0M
At eqm. conc. (0.35-x) M (0.40-x) M (x) M (x) M
Given: (0.35-x) = 0.19
x= 0.16 M
The expression for equilibrium constant for this reaction will be,
[tex]K_{eq}=\frac{[CO_2]\times [H_2]}{[CO]\times [H_2O]}[/tex]
Now put all the given values in this expression, we get :
[tex]K_{eq}=\frac{0.16\times 0.16}{(0.35-0.16)\times (0.40-0.16)}[/tex]
[tex]K_{eq}=\frac{0.16\times 0.16}{(0.19)\times (0.24)}=0.56[/tex]
Thus [tex]K_{eq}[/tex] at the temperature of the experiment is 0.56.
Please help will give brainliest
Perform the following
mathematical operation, and
report the answer to the correct
number of significant figures.
328 x 0.125 = [?]
Answers
Answer: 41.0
Explanation: When you multiply the two numbers you get 41 but you need to have the same amount of significant numbers as the number in the problem with the least significant numbers. I hope this helps
PLEASEEE PLEASEE HELP THIS IS MY FINAL EXAM AND I HAVE 30 MINS REMAINING
You have 5 moles of O2. How many moles of CO2, are produced?
Answers
Answer:
1
Explanation:
1234567891011121314151617181920
Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory as a primary standard. It has the unwieldy formula of KHC8H4O4. This is often written in shorthand notation as KHP. If 31.0 mL of a barium hydroxide solution are needed to neutralize 1.37 grams of KHP, what is the molarity of the barium hydroxide solution
Answers
Answer:
0.1082M of Barium Hydroxide
Explanation:
KHP reacts with Ba(OH)2 as follows:
2KHP + Ba(OH)2 → 2H2O + Ba²⁺ + 2K⁺ + 2P²⁻
Where 2 moles of KHP reacts per mole of barium hydroxide
To solve this question we must find the moles of KHP in 1.37g. With these moles and the reaction we can find the moles of Ba(OH)2 and its molarity using the volume of the solution (31.0mL = 0.0310L) as follows:
Moles KHP -Molar mass: 204.22g/mol-
1.37g * (1mol / 204.22g) = 0.006708 moles KHP
Moles Ba(OH)2:
0.006708 moles KHP * (1mol Ba(OH)2 / 2mol KHP) =
0.003354 moles Ba(OH)2
Molarity:
0.003354 moles Ba(OH)2 / 0.0310L =
0.1082M of Barium HydroxideIf the acid dissociation constant, Ka, for an acid HA is 8 x 104 at 25°C, what percent of the acid is dissociated
in a 0.50 M solution of HA at 25°C?
A
08%
Answers
Answer:
Percent dissociated = 3.92%
Explanation:
The equilibrium of the weak acid, HA, is:
HA ⇄ H⁺ + A⁻
Where Ka = 8x10⁻⁴ is: [H⁺] [A⁻] / [HA]
As both H⁺ and A⁻ comes from the same equilibrium we can write the concentrations of the species as follows:
[H⁺] = X
[A⁻] = X
[HA] = 0.50M - X
Where X is reaction coordinate
Replacing:
8x10⁻⁴ = X² / 0.50-X
4x10⁻⁴ - 8x10⁻⁴X - X² = 0
Solving for X:
X = -0.02M. False solution. There is no negative concentrations
X = 0.0196M. Right solution.
Replacing:
[A⁻] = 0.0196M
Percent of the acid that is dissociated is:
[A⁻] / [HA]₀ * 100
[HA]₀ is its initial concentration = 0.50M
0.0196M / 0.50M * 100
Percent dissociated = 3.92%What happens to the rate constant as activation energy increases?
A. The rate constant decreases.
B. The rate constant levels off.
C. The rate constant does not change.
D. The rate constant increases.
Answers
Answer:
A. The rate constant decreases
Explanation:
i took the quiz on a pex and it was correct
Really stuck on this question !! Pls help
Answers
Answer:
can not be determined
Explanation:
because when I tried to calculate it it didn't give me the answer options that was in the pic so it's definitely C
The sea, on average, has a molarity of 0.599 M NaCl. How many grams of NaCl is this for every 1 liter?
Pls answer
Answers
Answer:
it contains 0.599 g i hope it helps
Which best describes the conservation of energy as a pendulum swings in the path shown ?
A.The potential energy at point A is greater than the potential energy at point C.
B.The potential energy at point A is equal to the kinetic energy at point C.
C.The potential energy at point A is greater than the kinetic energy at point B.
D.The potential energy at point A is equal to the kinetic energy at point B.