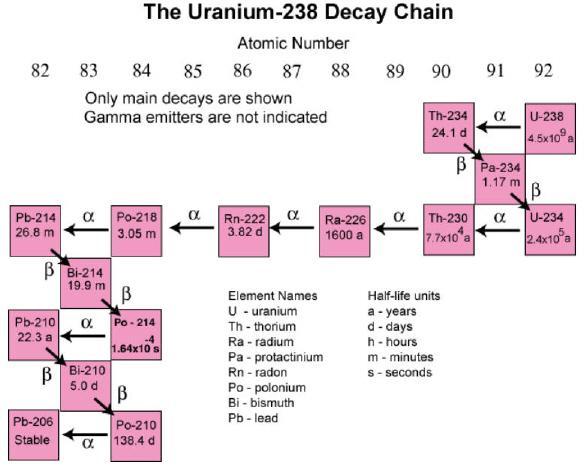
Answers
Answer:
can u explain a little more then i will help like what are the four
Explanation:
Answer:
d) U-238
Explanation:
edg 2021
Related Questions
what mix of chemicles gives fireworks thier color: explain for each color
Answers
Answer:
Metal salts commonly used in firework displays include: strontium carbonate (red fireworks), calcium chloride (orange fireworks), sodium nitrate (yellow fireworks), barium chloride (green fireworks) and copper chloride (blue fireworks).
Explanation:
Answer:
Metal salts commonly used in firework displays include: strontium carbonate (red fireworks), calcium chloride (orange fireworks), sodium nitrate (yellow fireworks), barium chloride (green fireworks) and copper chloride (blue fireworks).
The colors are produced by heating metal salts, such as calcium chloride or sodium nitrate, that emit characteristic colors. ... Barium – Barium is used to create green colors in fireworks, and it can also help stabilize other volatile elements. Calcium – Calcium is used to deepen firework colors
PLS HELP ASAP I ONLY HAVE 7 min
On which of the following factors does the amount of energy absorbed by an endothermic reaction depend?
1. Number of reactants
2. Physical state of the reactant
3. Sum of the potential energy of the reactants and products
4. Difference in the potential energy of the reactants and products
Answers
Answer:
Option 3 and 4
Explanation:
In an endothermic reaction, the potential energy of the system increase. The potential energy of the reactant is less as compared to the potential energy of the products and hence additional energy is required to produce products.
The potential energy of the reactants depend on their chemical state and not the physical state. Hence, option 2 is not correct. Also, the number of reactant do not make any significant impact on the determination of potential energy of reactants. Thus, option 1 is also incorrect
How many grams of Ni are formed from 55.3 g of Ni2O3?
2Ni2O3(s)⟶4Ni(s)+3O2(g)
Answers
Answer:
39.2 g
Explanation:
2Ni₂O₃(s) ⟶ 4Ni(s) + 3O₂(g)First we convert 55.3 grams of Ni₂O₃ into moles of Ni₂O₃, using its molar mass:
55.3 g ÷ 165.39 g/mol = 0.334 mol Ni₂O₃Then we convert 0.334 moles of Ni₂O₃ into moles of Ni, using the stoichiometric coefficients of the balanced reaction:
0.334 mol Ni₂O₃ * [tex]\frac{4molNi}{2molNi_2O_3}[/tex] = 0.668 mol NiFinally we calculate how much do 0.668 Ni moles weigh, using the molar mass of Ni :
0.668 mol Ni * 58.69 g/mol = 39.2 gWhich subatomic particles are transferred in a redox reaction?
Answers
Answer: Electrons
Explanation:
Commercial soaps are mixtures of ionic compounds typically made up of monatomic cations, such as Na and K , and organic polyatomic anions derived from fatty acids. These negatively charged molecular ions are characterized by the presence of hydrocarbon chains which are 12 to 18 carbon atoms long. How hard (solid, insoluble) or soft (liquid, soluble) a soap is depends on the nature of the anions and cations present in the system. Analyze how each of the following factors may affect the hardness or softness of soaps:
1. The nature of the cations. For example, Na* vs Li* vs K.
2. The length of the hydrocarbon chain. For example, 12 carbons (laureate lon), 14 carbons (myristate lon), or 18 carbons (stearate lon).
Answers
Answer:
Following are the solution to the given question:
Explanation:
For question 1:
The sodium soap containing Na+ is strong whereas the softer or liquids were potassium soap.It's hard to use lithium soap.These Na+, K+, and Li+ ions act as the hydrophilic center.Calcium and Magnesium ions could be substituted by hard water with increasing hydrophilicity.For question 2:
The hydrophobicity of its carbon chain increases but one appears weaker with only an increased length.Therefore, the laureate is hard, while the stearate is soft.differences between geometric isomerism and optical isomerism?
Answers
Geometric isomers have the same structural formulas but differ in the arrangement of groups at a single atom, at double bonds, or in rings. ... One of the optical isomers rotates the light in one direction, the other rotates the light in the opposite direction but by the same amount.
solve using the distributive property
- 592×7 + 592 × 3
Answers
Answer:
= -592 (7+3)
= 592 ×10
= -5920
hope it helps u
Mark me brainless
pls help me with is question.
Answers
Answer:
25cm³ = 0.025L of H2So4
Molarity of H2SO4 = 1moldm-³
Recall ... 1dm-³ = 1L
So the Molarity can also be 1mol/L
Mole = Molarity x volume in L
Mole of H2SO4 = 1mol/L x 0.025L
=0.025Moles of Sulphuric acid reacted.
From the equation of reaction
1mole of H2SO4 reacts to produce 1mole of Copper sulphate crystal
Since their Mole ratio is 1:1
It means that Since 0.025mole of H2SO4 reacted.... 0.025mole of CuSO4.5H2O would be produced
Nice.. OK
So we know the moles of CuSO4.5H2O produced
We can get the Mass
Recall
From
Mole=Mass/Molar Mass
Mass = Mole x Molar Mass
Molar Mass of CuSO4.5H2O = 64 + 32 + 16x4+ 5(2+16)
Mm= 250g/mol
Mass = 0.025mol x 250g/mol
= 6.25g of CuSO4.5H2O crystals Would be PRODUCED.
A molecule with 14 total electrons and 12 total protons
Answers
Answer:
Explanation:
Tenochtitlan was located on a swampy island in Lake Texcoco in what is today south central Mexico. The Aztecs were able to settle there because no one else wanted the land. At first, it wasn't a great place to start a city, but soon the Aztecs built up islands where they could grow crops. The water also worked as a natural defense against attacks from other cities.
Read more at: https://www.ducksters.com/history/aztec_empire/tenochtitlan.php
This text is Copyright © Ducksters. Do not use without permission.
The charge on a molecule with 14 electrons and 12 protons = -2
Although your question is vague a general answer is provided above
When the number of protons and the number of electrons in a molecule are equal to each other, the charge on the molecule will be neutral, this is because Electrons are negatively charged while protons are positively charged.
Therefore a molecule with 14 electrons and 12 protons will have a charge of
= -14 + 12 = - 2
learn more : https://brainly.com/question/18169295
What is the volume, in cubic meters, of an object that is 0.21 m long, 4.7 m wide, and 5.3 m high?
Answers
Answer:
The formula for volume of a rectangle is length multiply by width multiply thus, 0.25 m multiply 6.1 m multiply by 4.9 m = 7.5m^3.
Explanation:
the least number of significant figures is 2 thus the final answer will have the same number of significant figures. 7.5m^3
by what process does water vapor become a cloud?
a. evaporation
b. transpiration
c. condensation
d. precipitation
Answers
Determine what mass of carbon monoxide and what mass of hydrogen are required to form 6.0 kg of methanol by the reaction CO(g) + 2H2(g) -> C H3OH(l)
Answers
Answer:
5250 grams or 5.25 kg of carbon monoxide and 375 grams of hydrogen are required to form 6 kg of methanol.
Explanation:
The balanced reaction:
CO (g) + 2 H₂ (g) -> CH₃OH (l)
By stoichiometry of the reaction, the following amounts of moles of each compound participate in the reaction:
CO: 1 moleH₂: 2 molesCH₃OH: 1 moleBeing the molar mass of each compound:
CO: 28 g/moleH₂: 1 g/moleCH₃OH: 32 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
CO: 1 mole* 28 g/mole= 28 gramsH₂: 2 moles* 1 g/mole= 2 gramsCH₃OH: 1 mole* 32 g/mole= 32 gramsBeing 6 kg equivalent to 6000 grams (1 kg= 1000 grams), you can apply the following rules of three:
If by stoichiometry 32 grams of methanol are formed from 28 grams of carbon monoxide, 6000 grams of methanol are formed from how much mass of carbon monoxide?[tex]mass of carbon monoxide=\frac{6000 grams of methanol*28 grams of carbon monoxide}{32 grams of methanol}[/tex]
mass of carbon monoxide= 5250 grams= 5.25 kg
If by stoichiometry 32 grams of methanol are formed from 2 grams of hydrogen, 6000 grams of methanol are formed from how much mass of hydrogen?
[tex]mass of hydrogen=\frac{6000 grams of methanol*2 grams of hydrogen}{32 grams of methanol}[/tex]
mass of hydrogen= 375 grams
5250 grams or 5.25 kg of carbon monoxide and 375 grams of hydrogen are required to form 6 kg of methanol.
A 5.0 L sample of gas at 300. K is heated to 600. K. What will the new volume of the gas be?
Answers
Answer:
[tex]V_2=10L[/tex]
Explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the required new volume by using the Charles' law as a directly proportional relationship between temperature and volume:
[tex]\frac{V_2}{T_2} =\frac{V_1}{T_1}[/tex]
In such a way, we solve for V2 and plug in V1, T1 and T2 to obtain:
[tex]V_2=\frac{V_1T_2}{T_1}\\\\V_2=\frac{5.0L*600K}{300K}\\\\V_2=10L[/tex]
Regards!
How many sigma and pie bonds are in the following compound?
1.00
on
H:0:
| ||
H-C-C-H
1
H
Answers
For the first
H : O : H
Theres two bonds here
Each other are sigma
So 2Sigma bonds present
For the Second
I believe you wanted to write Ethyne....
In a triple bond... Theres 1sigma and 2 Pi bonds
Every single bond is a sigma bond
So
Counting all
we have
2Pi bonds and 3Sigma bonds
balance the equation P + O2 → P4O10
Answers
which shows a disaccharide
Answers
Answer: It is :B:
Explanation: also sub to technoblade
Control rods in nuclear reactors are made of materials that absorb free neutrons in order to
slow down the chain reaction.
True
False
Answers
A chemical equilibrium exists when: A chemical equilibrium exists when: there are equal amounts of reactants and products. the rate at which reactants form products is the same as the rate at which products form reactants. the sum of reactant and product concentrations equals one mole. reactants are completely changed to products. the rate at which reactants form products becomes zero.
Answers
Answer: A chemical equilibrium exists when the rate at which reactants form products is the same as the rate at which products form reactants.
Explanation:
When the concentration of both the reactants and products do not change with time then it means the chemical reaction has reached to a state of chemical equilibrium.
For example, [tex]CO(g) + Cl_{2}(g) \rightleftharpoons COCl_{2}(g)[/tex]
Therefore, we can conclude that a chemical equilibrium exists when the rate at which reactants form products is the same as the rate at which products form reactants.
PLEASE HELP ME!
WILL MARK BRAINLIEST
Water Cycle
Listed in the Item Bank are some important labels for sections of the image below. To find out more information about labels, some have more details available when you click on them. Drag and drop each label to the corresponding area it identifies in the image.
Answers
The one on the top left corner is condensation. The one below that is evaporation. The one below that in the water is accumulation. The one next to accumulation is ground flow. The one on the trees is transpiration. The one above transpiration is surface flow. The one above surface flow is precipitation and above precipitation it’s condensation. Lmk if you were able to get that.
how many atoms of potassium are in 4.25 g of potassium
Answers
Answer:
6.55 x 10^22 atoms K
Explanation:
Convert from grams to moles (using K molar mass)
K molar mass = 39.098 g/mol
4.25 g K x 1 mol K / 39.098 g K
[grams cancel out]
= 0.1087 mols K
Convert from moles to atoms (using Avogadro's number)
Avogadro's number = 6.0221 x 10^23
0.1087 mols K x 6.0221 x 10^23 atoms K / 1 mols K
[mols cancel out]
= 6.545987 x 10^22
[with proper significant digits.....]
= 6.55 x 10^22 atoms K
To lower the chance of suffering from decompression sickness (the bends), scuba divers use a mixture of gases in their air tank (typically oxygen and nitrogen gas in recreational dives). Assuming no other gas is present besides oxygen and nitrogen, if the mole fraction of oxygen present is 0.21, what is the partial pressure of nitrogen gas if the total pressure is 111.7 atm
Answers
Answer: The partial pressure of nitrogen gas if the total pressure is 111.7 atm is 88.243 atm.
Explanation:
Given: Mole fraction of oxygen = 0.21
Total pressure = 111.7 atm
It is known that the sum of moles fractions is always equal to 1. So, mole fraction of nitrogen is calculated as follows.
Mole fraction of nitrogen + mole fraction of oxygen = 1
Mole fraction of nitrogen = 1 - mole fraction of oxygen
Mole fraction of nitrogen = 1 - 0.21
Mole fraction of nitrogen = 0.79
Now, formula used to calculate the partial pressure of nitrogen is as follows.
[tex]P_{N} = X_{N} \times P_{total}[/tex]
where,
[tex]P_{N}[/tex] = partial pressure of nitrogen
[tex]X_{N}[/tex] = mole fraction of nitrogen
[tex]P_{total}[/tex] = total pressure
Substitute the values into above formula as follows.
[tex]P_{N} = X_{N} \times P_{total}\\= 0.79 \times 111.7 atm\\= 88.243 atm[/tex]
Thus, we can conclude that the partial pressure of nitrogen gas if the total pressure is 111.7 atm is 88.243 atm.
When the ball is at rest, what forces are acting on it?
Answers
The oceanic crust is destroyed at convergent boundaries because it_______________________ *
a) diverges to form a rift valley
b) goes into a subduction zone where it is melted by the hot magma
c) transforms
d) the rock crumbles at an ocean ridges
Answers
Answer: I believe the answer is d) the rock crumbles at an ocean ridges
Explanation:
Which base(s) is weaker than ammonia?
hydroxylamine, methylamine, and pyridine
pyridine only
hydroxylamine and methylamine
hydroxylamine and pyridine
Answers
Answer:
Hydroxylamine and pyridine
Explanation:
Just did it
For many purposes we can treat butane as an ideal gas at temperatures above its boiling point of . Suppose the pressure on a sample of butane gas at is cut in half. Is it possible to change the temperature of the butane at the same time such that the volume of the gas doesn't change? yes no If you answered yes, calculate the new temperature of the gas. Round your answer to the nearest °C.
Answers
Answer:
A. Yes
B. The new temperature of the gas is -116 °C
Note: The question is incomplete. The complete question is given below :
For many purposes we can treat butane C H10) as an ideal gas at temperatures above its boiling point of - 1. °C. Suppose the pressure on a 500 mL sample of butane gas at 41.0°C is cut in half. Iyes Is it possible to change the temperature of the butane at the same time such that the volume of the gas doesn't change? yes no If you answered yes, calculate the new temperature of the gas. Round your answer to the nearest °C.
Explanation:
A. According to the pressure law of gases,for a fixed mass of gas the pressure of a gas is directly proportional to its Kelvin temperature once the volume is kept constant. This means that a change in temperature can bring about a change in pressurein a gas at constant volume.
B. From the pressure law of gasese: P1/T1 = P2/T2
Where initial pressure = P1, final pressure = P2
Initial temperature = T1, final temperature = T2
For the butane gas;
P1 = P
P2 = P/2
T1 = 41°C = (273 + 41 ) K = 314 K
T2 = ?
From the equation, T2 = T1 × P2 / P1
T2 = 314 × P/2 /P
T2 = 157 K
T2 = (157 - 273) °C = -116 °C
Therefore, the new temperature of the gas is -116 °C
Andrea is making a poster to show how the nervous system uses signals to send information through the body. These signals are sent through nerves. What parts should Andrea include in her diagram of a nerve as evidence to support her explanation?
neurons only
neurons, axons, and dendrites
dendrites, blood vessels, and muscle cells
axons, blood vessels, and connective tissue
Answers
Answer:b
Explanation:
In a receptacle we have 29 g of hydrochloric acid that react with an excess of ammonia according the following equation:
HCl + NH3 → NH4Cl
The percent yield of the reaction is 54 %. Determine:
1.Mass of ammonium chloride obtained.
2.How many moles of ammonium chloride are forme
Answers
Answer:
2969429th Ave
Explanation:
i dont know
HELLP ME PLSSS
The passing of heat through a material is called ________.
A. vibration
B. conduction
C. radiation
D. convectio
Answers
Answer:
B
Explanation:
conduction is the transfer of heat between objects that touch
Uranium is an element with three naturally occurring isotopes: 238U, 235U, and 234U. This means that 238U, which has a mass number of 238 has _______more than 235 u which has a mass number of 235.
Answers
Answer:
The correct answer is - neutrons.
Explanation:
Uranium has various isotopes found naturally that are three 238U, 235U, and 234U. Uranium has an atomic number of 92 which means there are 92 protons and 92 electrons in the atomic structure.
Isotopes have the same number of protons but a different number of neutrons that can vary from 141 to 146. U-238 has 146 neutrons in the nucleus, whereas 235 U has 143 neutrons.
The U- 238 has more neutrons than U- 235. Atomic mass is the sum total of the nucleons or protons and neutrons.
What are Isotopes?
They are the different variants of the same molecules which have the same number of protons but the different number of neutrons.
Atomic mass is the sum total of the nucleons or protons and neutrons. The atomic number of Uranium is 92, the rest of the mass comes from neutrons.
Therefore, the U- 238 has more neutrons than U- 235.
Learn more about Isotopes:
https://brainly.com/question/9099776
Calculate the ionization constant for the following acids or bases from the ionization constant of its conjugate base or conjugate acid: Keep your answer to 2 significant figures (CH3)3NH+
Answers
Answer:
7.41 × 10⁻⁵
Explanation:
Let's consider the basic dissociation reaction of trimethylamine (CH₃)N).
(CH₃)N + H₂O = (CH₃)NH⁺ + OH⁻
According to Brönsted-Lowry, in this reaction (CH₃)N is a base and (CH₃)NH⁺ is its conjugate acid. The pKb for (CH₃)N is 9.87. We can calculate the pKa of (CH₃)NH⁺ using the following expression.
pKa + pKb = 14
pKa = 14 - pKb = 14 - 9.87 = 4.13
Then, we can calculate the acid dissociation constant for (CH₃)NH⁺ using the following expression.
pKa = -log Ka
Ka = antilog - pKa = antilog -4.13 = 7.41 × 10⁻⁵