Answers
Answer:
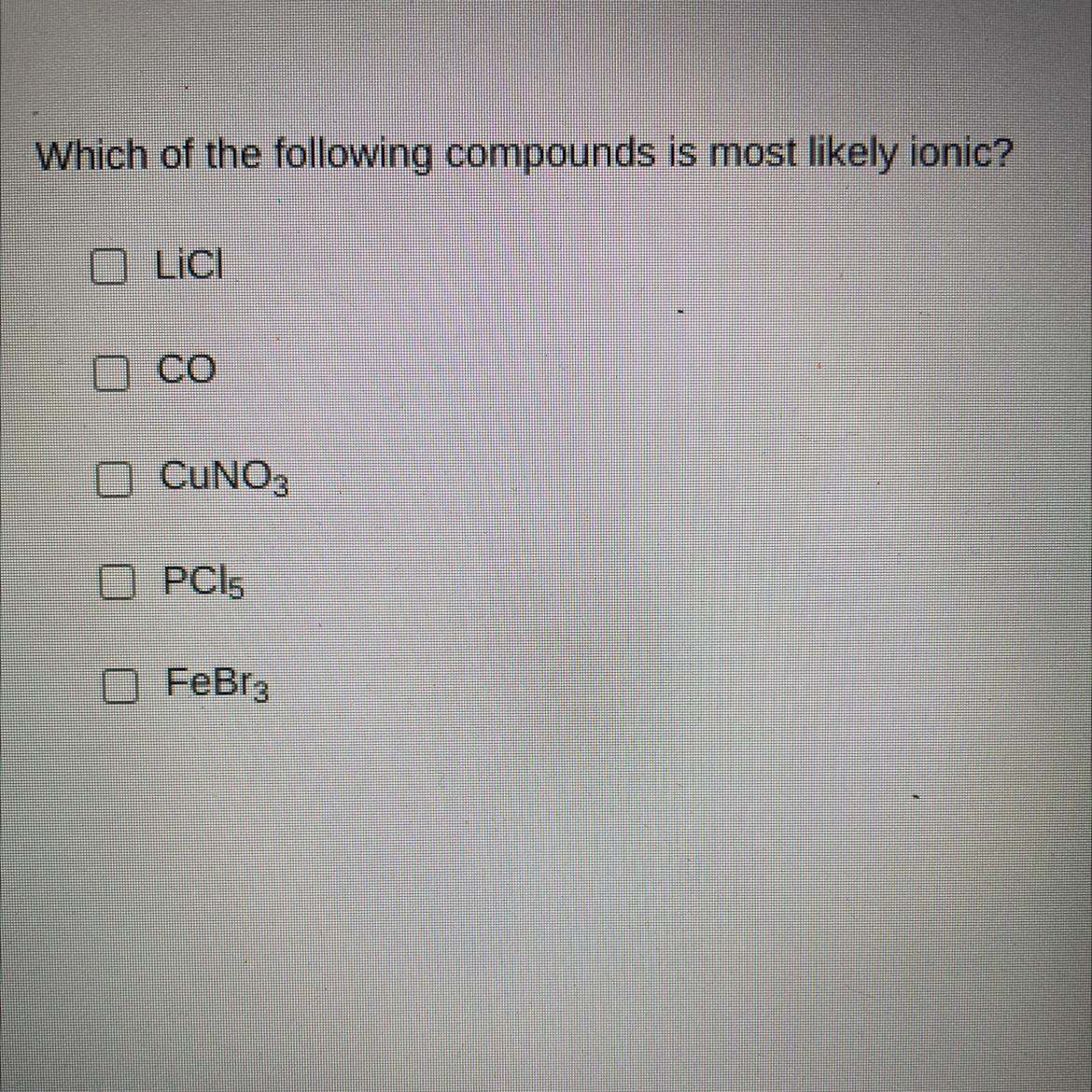
LiCl.
Explanation:
Hello there!
In this case, since it is known that the ionic compounds are specially formed between metals and nonmetals, it is possible for us to discard CO and PCl5 as they have both nonmetals.
On the other hand, since the bonds between nonmetals and transition metals like iron and copper may not be completely ionic due to the electronegativity trend, we infer that the most likely ionic compound is LiCl because of the large electronegativity difference.
Regards!
Related Questions
Name one trend observable in a periodic table
Answers
Ionization energy
Electron affinity
Atomic radius
Melting point
Answer:
Major periodic trends include: electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character.
Explanation:
These six are the major trends.
b. What three products are necessary in order for a hydrocarbon combustion reaction to take place?
Answers
Explanation:
Description. Regardless of the type of hydrocarbon, combustion with oxygen produces 3 products: carbon dioxide, water and heat, as shown in the general reaction below.
If a balloon expands to occupy a volume of 5.25 Liters going from 15 atm to 25 atm, what was the initial volume?
03.15L
O 7.75L
Answers
Answer:
V₁ = 8.75L
Explanation:
P₁ × V₁ = P₂ × V₂
15atm × V₁ = 25atm × 5.25L
V₁ = 8.75L
All of the following are advantages of nuclear power EXCEPT: uranium mines cause less environmental damage than coal mines because less uranium is needed to generate power. nuclear power plants generate no nitrogen oxides and sulfur dioxide. uranium generates far greater amounts of energy than coal by weight or volume. the power-generating process is emission-free. nuclear wastes can be safely disposed of.
Answers
Answer:
nuclear wastes can be safely disposed of
Explanation:
In comparing fossil fuel power plants with nuclear power plants, it is obvious that fossil fuel power plants lead to a large volume of emission of oxides of carbon and sulphur.
Also, a larger volume of coal needs to be burnt to generate energy compared to a minute amount of uranium fuel that can sustain a nuclear power plant for a long period of time thereby reducing the environmental damage associated with mining of the fuel.
However, the problem of nuclear waste disposal have remained a thorn in the flesh. It is often difficult to safely dispose of spent uranium fuel. This is a major disadvantage of the use of nuclear power.
Sea turtles use the earths geomagnetic filed to navigate their way home. What is the reason earth has a geomagnetic filed?
Answers
Answer:
Due to the iron inside the earth.
Explanation:
Imagine a sphere of Iron, as big as two-third the size of the moon and as hot as 5700 Kelvin. That is the Earth’s core.
The iron core isn't in its liquid form even at that temperature because it is crushed under immense gravity. This core is surrounded by 2000 km of other metals like iron and nickel which are in their molten state.
The temperature is not the same at every point in this molten layer. The hotter and less dense matter rises up, and the warm denser matter sinks. This causes convectional currents in the interior of the Earth.
Because of the Earth’s spin, there is a force that is established called the Coriolis force which causes swirling whirlpools here too.
This flow of molten metals produces electric currents which generate self-sustaining magnetic fields. And as a result of the Coriolis force, all these combined effects add up to produce one big magnetic field engulfing the Earth aligned in one direction.
Calculate the freezing point of a solution made by dissolving 13 g potassium sulfide in 150 g H2O Kf for water = 1.86 C/m
Answers
Answer:
[tex]T_F=-4.4\°C[/tex]
Explanation:
Hello there!
In this case, according to the given information, it is possible for us to calculate the freezing point of an aqueous solution of potassium sulfide by using the following equation:
[tex]T_F=T_{solv}-i*m*Kf[/tex]
In such a way, we firstly calculate the molality of this solution according to:
[tex]m=\frac{\frac{13g}{110.262 g/mol} }{0.150kg} =0.79m[/tex]
Finally, since the Van't Hoff's factor for K2S is 3, the freezing point of the solution turns out to be:
[tex]T_F=0\°C-3*0.79m*1.86\°C/m\\\\T_F=-4.4\°C[/tex]
Regards!
Which chemical equation is balanced as written?
PLS ANSWER FAST
Answers
Answer:
THERE ANSWER IS C
Explanation:
A quantity of gas was cooled from 323 K to 273 K. Express this temperature
difference in degrees Celsius and Kelvins. What do these temperatures
represent about the gas particles? Which temperature scale is a more accurate
depiction of this representation? Explain briefly.
Answers
Answer:
give more details
Explanation:
A sample of ideal gas at room temperature occupies a volume of 10.0 L at a pressure of 302 torr. If the pressure changes to 1510 torr
with no change in the temperature or moles of gas, what is the new volume, V2?
Answers
Explanation:
boyles law; P1V1=P2V2
302×10=1510×V2
V2=2L
Which statement best describes thermodynamics?
A. The study of how energy changes and moves
B. The study of physical laws acting on matter
C. The study of electricity at low temperatures
D. The study of intermolecular forces in liquids
Answers
Answer:
a
Explanation:
how many moles of CO2 are in 35.0 liters of CO2 at STP?
Answers
Answer:
75300g ..........................
How many grams of gas are present at 221 mL of Ar and 253 torr at -27 degree Celsius
Answers
Answer:
immma send you the link friend me on hereExplanation:
A substance has a pH of 4. Is it an acid or a base?
Answers
14. Simply, explain the role of both the nucleus and the ribosome in protein synthesis.
Answers
Answer:
Eukaryotic cells have a true nucleus, which means the cell's DNA is surrounded by a membrane. Therefore, the nucleus houses the cell's DNA and directs the synthesis of proteins and ribosomes, the cellular organelles responsible for protein synthesis.
Ribosomes are the sites in a cell in which protein synthesis takes place. ... Within the ribosome, the rRNA molecules direct the catalytic steps of protein synthesis — the stitching together of amino acids to make a protein molecule.
When a liquid is cooled kinetic energy of the particles remain the same, increase or decrease
Answers
Answer:
Decreases.............
Answer:
kinetic energy of the particles decreases
Explanation:
just remember, cold = decrease and hot = increase
Measure the volume of the gas in the syringe.
Estimate it to the nearest 0.5 mL. mL
30.5
Intro
32.5
37.5
40.0
Answers
Answer: 47.5
Explanation:
Answer: 47.5 is your answer
Explanation:
good luck on the rest XoXo
HELP ME PLEASE I AM STRUGGLING
Answers
maybe umm think so pick think so
HELP!!!!!! PLEASE!!!
Answers
Answer:
C
Explanation:
sorry if im wrong!!
What are some of the common characteristics for the elements in a group? Use examples to support your explanation.
Answers
Answer:
The elements in each group have the same number of electrons in the outer orbital. Those outer electrons are also called valence electrons. They are the electrons involved in chemical bonds with other elements
Explanation:
Elements are the simplest complete chemical substances. Each element corresponds to a single entry on the periodic table. An element is a material that consists of a single type of atom. Each atom type contains the same number of protons.
mplete the following Charles' Law calculations. If the current temperature is 25 degrees C and you have a 2L balloon, identify the new volume of the balloon if you increase the temperature to 30 degrees C. Remember to change Celsius to Kelvin: K= C + 273.
Answers
Answer:
New volume = 2.03 L
Explanation:
Given that
Initial temperature, T₁ = 25°C = 298 K
Initial volume, V₁ = 2L
Final temperature, T₂ = 30°C = 303 K
We need to find the final volume. The mathematical form of Charle's law is given by :
[tex]\dfrac{V_1}{V_2}=\dfrac{T_1}{T_2}\\\\V_2=\dfrac{V_1T_2}{T_1}[/tex]
Put all the values,
[tex]V_2=\dfrac{2\times 303}{298}\\\\V_2=2.03\ L[/tex]
so, the new volume is equal to 2.03 L.
Can someone please help me with this question
Answers
Explanation:
a) 3Pb(NO3)2 + Al2(SO4)3 ---> 3PbSO4 + 2Al(NO3)3
Double displacement
b) 2Cl2 + 3O2 ---> 2Cl2O3
Synthesis
c) 2Fe2O3 + 3C ---> 4Fe + 3CO2
single displacement
What type of reaction is illustrated?
C3H8 +502 + 3C02 + 4H2O
А
B
decomposition
combustion
single
replacement
Answers
Answer:
Combustion
Explanation:
True or False When one side of a molecule is electronegative (δ-) and the other side of the
molecule is electropositive (δ+), it is said to have a dipole moment. Water has
a dipole moment.
Answers
Answer:
True; When one side of a molecule is electronegative (δ-) and the other side of the
molecule is electropositive (δ+), it is said to have a dipole moment.
Explanation:
A dipole moment exists in a molecule as a result of differences in the electronegativity values between the atoms of the elements involved in the chemical bonding.
When a strogly electronegative atom such as oxygen or chlorine is chemically bonded to a less electronegative or an electropositive atom such as hydrogen, there is an uneven sharing of the electrons involved in the bonding. The more electronegative atoms tends to draw the shared electrons mostly to themselves. This induces a partially negative charge (δ-) on them while leaving the electropositive atoms with a partially positive charge (δ+).
Water is an example of a molecule having a dipole moment. The oxygen atoms are more electronegative than hydrogen and as such draw the shared electrons to themselves more, inducing a partial positive charge (δ+) on the hydrogen atoms while they themselves develop a partial negative charge (δ-).
Which represents the greatest number of phosphorous
atoms?
Answers
Answer:
Helium has the most number of mol out of all the options and will thus have the highest number of atoms.
Explanation:
2L of hydrogen has an initial pressure of 750 mmHg, what is the final pressure in mmHg if the volume increases to 20 L with a constant temperature of 37 degrees C?
Answers
Answer: The final pressure is 75 mm Hg.
Explanation:
According to Boyle's law, at constant temperature the pressure of a gas in inversely proportional to volume.
Since, it is given that the temperature is constant. Hence, formula used is as follows.
[tex]P_{1}V_{1} = P_{2}V_{2}[/tex]
Substitute the values into above formula as follows.
[tex]P_{1}V_{1} = P_{2}V_{2}\\750 mm Hg \times 2 L = P_{2} \times 20 L\\P_{2} = \frac{750 mm Hg \times 2 L}{20 L}\\= 75 mm Hg[/tex]
Thus, we can conclude that the final pressure is 75 mm Hg.
A solution is prepared by mixing 2.50g of CaCl2 with 50.0g H2O, what is the mass percent of CaCl2?
A. 3.76%
B. 4.76%
C. 5.76%
D. 6.76%
Answers
Hi, what are you doing?
Answers
Answer:
i am dancing and you ok hom
Bananas Foster is an example of a dessert that is flambéed. A Bananas Foster label states the accepted number of Calories to be only 300 calories, but a calorimetry experiment measured there to be 318 calories. Calculate the percent error.
Answers
Answer:
6.00%
Explanation:
Step 1: Given data
Accepted value for the number of calories in a Bananas Foster: 300 calories
Measured value for the number of calories in a Bananas Foster: 318 calories
Step 2: Calculate the percent error in the measure
We will use the following expression.
%error = |accepted value - experimental value|/ accepted value × 100%
%error = |300 cal - 318 cal|/ 300 cal × 100% = 6.00%
how long did it take for the moon to go around earth
Answers
Answer: about one month
Explanation:
PLZ HELP ON TIMER WILL GIVE BRAINLIEST
How does technology limit the future of space exploration?
There are too many devices in space interfering with taking correct measurements.
Scientists cannot make contact with older satellites in outer space.
Scientists are able to work both with current and future technology.
Scientists must first develop certain technologies before missions can be completed.
Answers
Answer:
I would put the final answer choice: "Scientists must first develop certain technologies before missions can be completed"
Explanation:
The first option is partially true, but we have ways around it.
The second option is straight-up false.
The third option doesn't make much sense, how can one work with technology that will be developed in the future and doesn't yet exist?
Therefore, the fourth option is the best.
Hope this helps
-cyber